Additional Capabilities
Including assay development, manual patch clamp, sustainability and investigative research.
Manual patch-clamp
Manual patch-clamp (MPC) was developed by Erwin Neher and Bert Sackman in 1976 and subsequently awarded a nobel prize in 1991. Facilitating recording of electrical events from single cells in real-time, MPC provides a powerful and versatile technique which has revolutionised our understanding of cardio- and neurophysiology.
Cultured cells or ex vivo tissue preparations are perfused in a recording chamber attached to a microscope, allowing the user to visually select cells of interest. Glass microelectrodes are positioned at the cell membrane and suction is applied to form an electrically tight seal. Summated electrical events can be recorded in cell-attached or whole-cell configurations (Figure 1), or alternatively small patches of membrane can be isolated and single ion-channel events recorded (outside- or inside-out configurations).

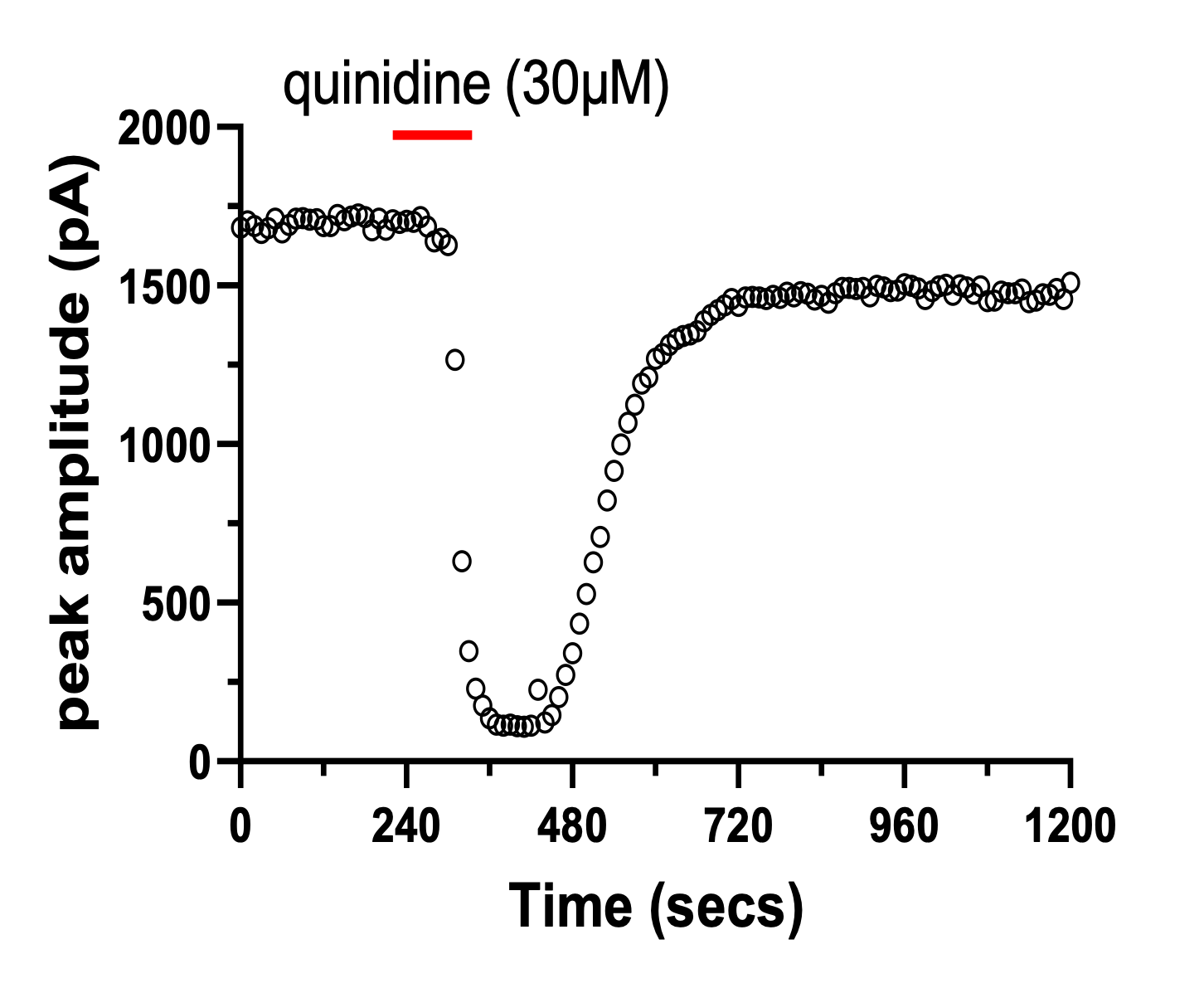
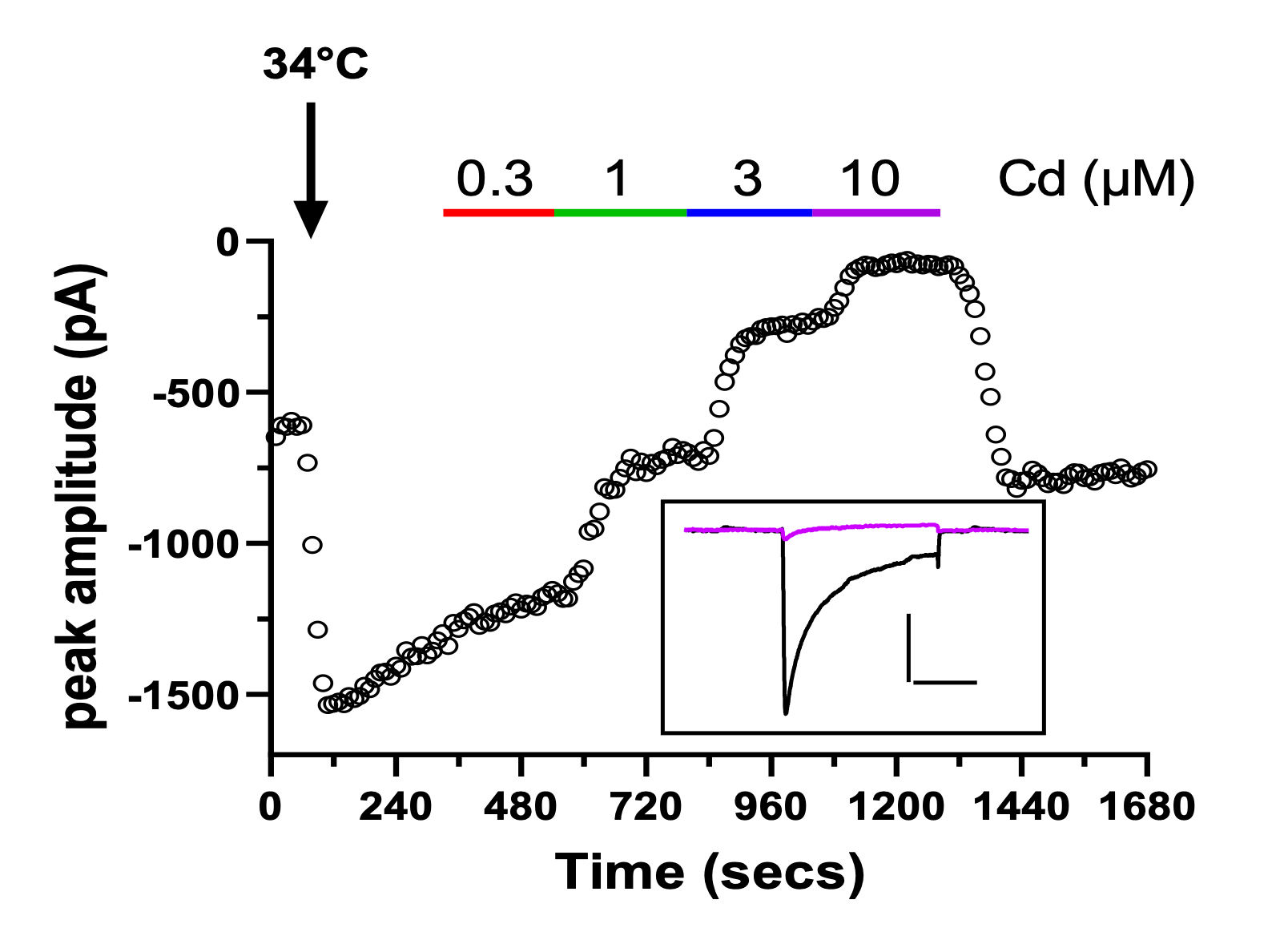
Figure 1 – example time course plots and corresponding traces (insets) recorded from cells stably expressing hERG (A, scale bar 500pA, 500ms) or CaV2.2 (B, scale bar 500pA, 20ms) channels.
A key drawback of MPC is that its low-throughput nature is unconducive to screening large numbers of compounds quickly. Resultantly automated patch-clamp (APC) machines were developed and have largely replaced MPC as the “go-to” technique for ion channel screening. Despite this, MPC affords many advantages, for example:
- Studying the phenotypes of fluorescently tagged transgenes (Figure 2).
- Single channel recordings
- GLP studies
- Assay development
- Assessing the intracellular actions of low-volume compounds.
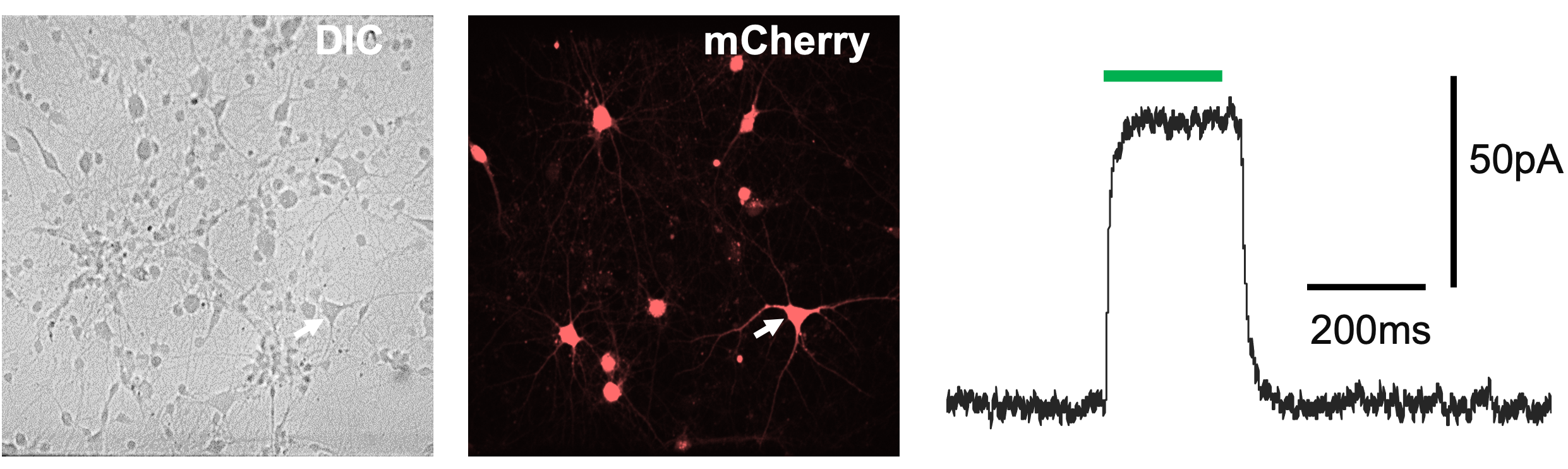
Figure 2– whole-cell MPC recording of fluorescently tagged cell (shown by white arrow)
ApconiX offers both MPC and APC for custom studies tailored to our clients needs.
Measurement of recovery from block in voltage-gated sodium channels and Vaughan-Williams classification
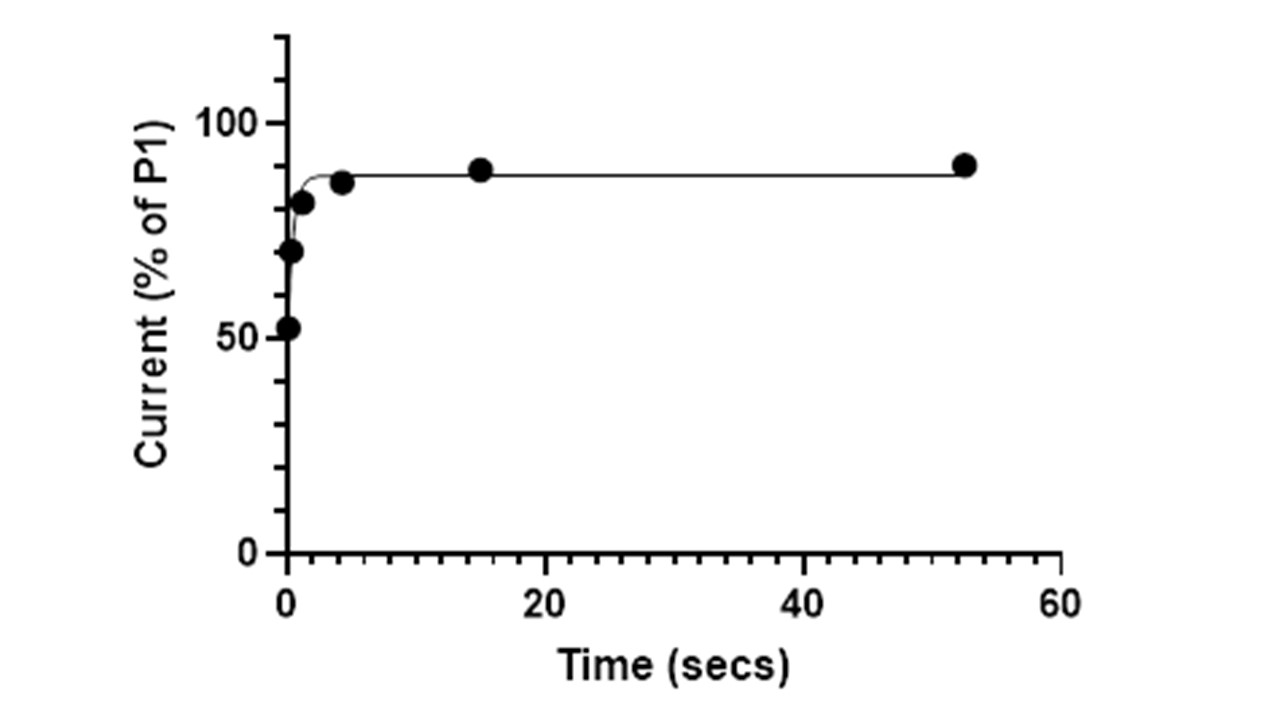
Graph showing recovery from sodium channel block, 30uM mexiletine
The understanding of cardiac arrythmias is critical to drug discovery. Decades of work has increased our understanding of the role of hERG potassium channel in QT prolongation and testing strategies have evolved to identify and reduce the liability through lead optimisation (Pollard et al., 2008). More recently, the CiPA paradigm has expanded the focus to a wider panel of cardiac ion channels, coupled with in silico modelling and human cardiomyocyte studies to define the pro-arrythmic risk (Gintant et al., 2016).
Beyond this, the FDA have become increasingly interested in the functional consequences of blocking the cardiac sodium channel. It is now requesting studies to define the recovery from block and classify drugs according to the Vaughan-Williams scheme (Lei et al., 2018).
In our laboratory we are able to measure recovery from block by automated patch-clamp. The test item is studied alongside quinidine, mexiletine and flecainide which show fast, medium and slow recovery kinetics, respectively.
Experiments are performed at near physiological temperature. These studies are non-GLP but can be used for regulatory submission.

Sustainability at ApconiX
As an environmentally conscious company, we are always looking for ways to make sustainability an integral part of everyday lab work, and to reduce plastics waste where we can. Each year the consumables and equipment required for day-to-day cell culture generates tons of plastic waste, which cannot be reused or recycled. This is a long-standing issue which impacts the environment by adding to the plastic waste sent to landfill, and increases the cost of running a lab. Our work in ion channel screening means that our lab relies heavily on single-use plastics in our day-to-day operations, which carry a financial and environmental cost and contribute to our carbon footprint.
It was estimated that up until 2022, each month our cell culture department would generate an average of 80Kg consumables and plastic waste, in addition to which most recycling plants don’t accept plastics waste from labs due to contamination.
Over the last 6 months we have conducted a study where we generated assay ready cell lines in place of continued cell culture, which has been known to save time, and reduce the amount of plastics waste generated compared with traditional cell culture methods. These are cells frozen at high viability, making them fit for drug safety screening, and can be used in patch-clamp assays directly after thawing without culture or passage. The assay ready cells have shown excellent viability and functional activity comparable to live cultured cells following dose response testing.

Reducing the use of fetal bovine serum in cell culture
Fetal bovine serum (FBS) is the liquid fraction of blood harvested from bovine fetuses. It’s commonly used as a serum-supplement in cell culture media as it contains thousands of components conducive to cell attachment, growth, and maintenance. However, the specific composition of FBS is largely undefined and this has led to several scientific problems. For example, FBS may vary between batches (affecting experimental variability) and contain adverse factors (raising issues of biosafety). There are also ethical concerns surrounding the practice of fetal blood harvesting as it exposes the fetus to pain and discomfort.From a financial viewpoint, the demand and unsteady supply of FBS has led to prices rocketing by over 300% in recent years, making it a significant cost-driver of cell culture media. It would be scientifically, ethically, and financially favorable to use a chemically defined synthetic alternative, but a serum-free medium with comparable efficiency is yet to be developed. These experiments aimed to adapt our cell lines to serum reduced conditions using commercially available media and supplements.
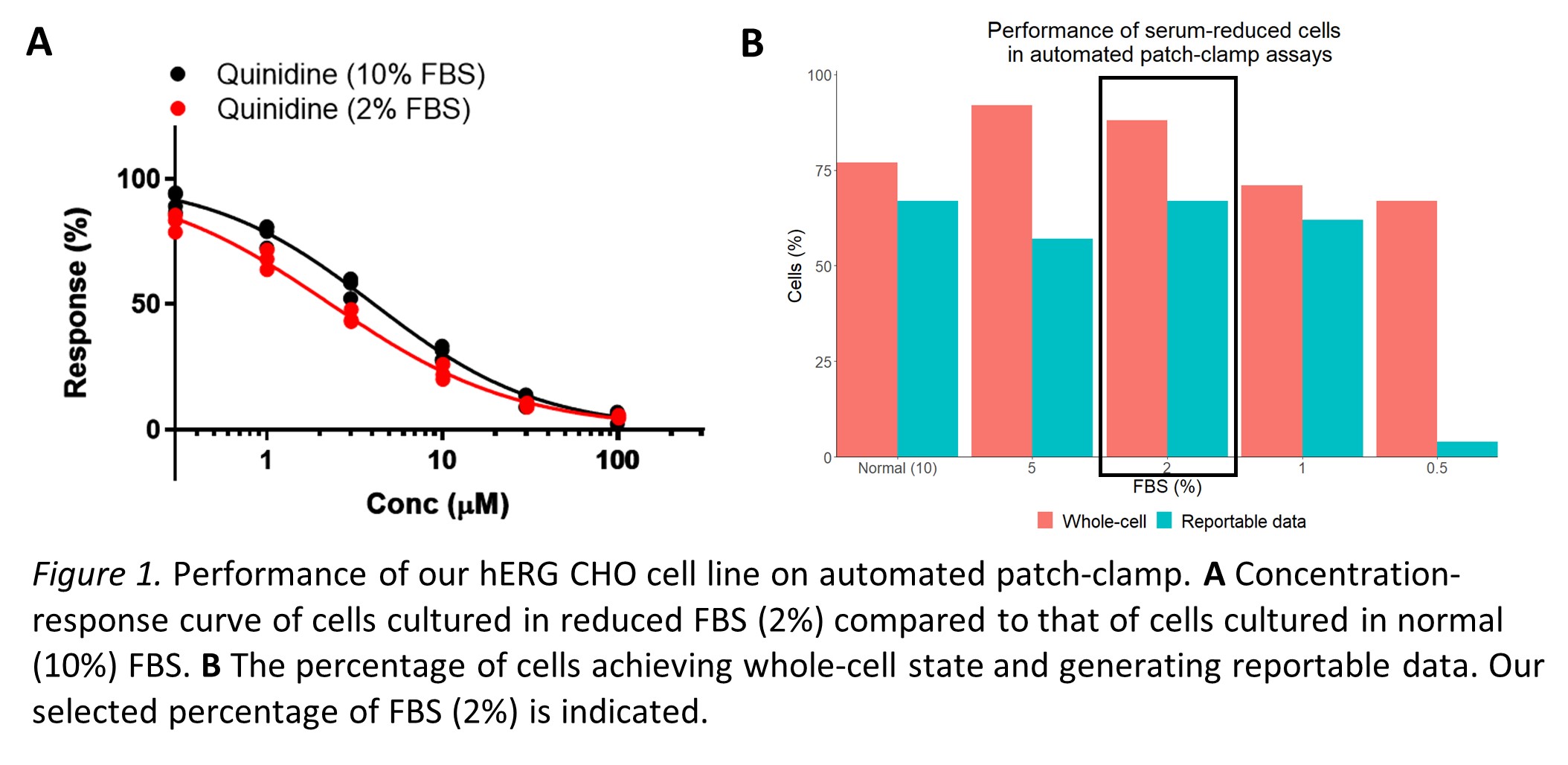
FBS reduction was investigated using our hERG CHO cell line. These cells were cultured in several percentages of FBS, and their performance was measured via automated patch-clamp (figure 1). We concluded that a reduction from 10% to 2% was the most successful adaptation. With efforts underway to optimize and validate their performance in this assay, the lab is excited to continue working towards a more ethical and sustainable approach to cell culture.

